Oral Cannabis consumption and intraperitoneal THC:CBD dosing results in changes in brain and plasma neurochemicals and endocannabinoids in mice | Journal of Cannabis Research
Chemicals, standards, and reagents
Whole leaf Cannabis sativa, purified THC, and purified CBD were obtained from National Institutes of Drug Abuse (NIDA) under a DEA Schedule I license to Dr. Reisdorph. The Low THC (< 1%) / Very High CBD (> 10%) strain of C. sativa was utilized. Purified THC was provided as 10/mg/ml (-)-trans-Delta-9-THC in ethyl alcohol 95% and dried, synthetic CBD was reconstituted in 200 proof ethanol. Standards, chemicals and reagents for extraction and LC/MS analysis were of LC/MS or HPLC-grade. Ethanol (200 proof), Sodium Hydroxide (NaOH) and Sodium Chloride (NaCl) were purchased from Fisher Scientific (Fair Lawn, NJ). LC/MS water was purchased from Honeywell Burdick & Jackson (Muskegon, MI). Tween80 and sodium chloride was purchased from MilliporeSigma (St. Louis, MO). Cannabis plant material, THC and CBD standards were provided by NIDA (Bethesda, MD).
Mice
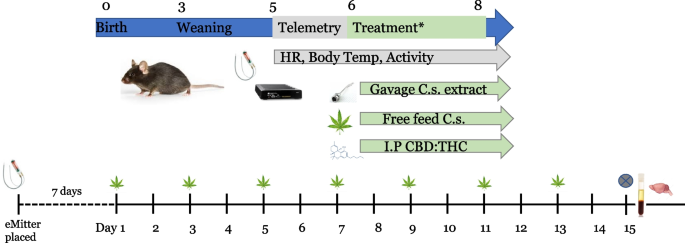
All mouse studies were approved by the University of Colorado Institutional Animal Care and Use Committee (IACUC). Male C57BL/6 J mice (Jackson Laboratories, Bar Harbor, ME) were maintained on a standard diet throughout the study. Mice were housed 1 per cage for mice receiving telemetry devices and no more than 2 per cage for mice not receiving telemetry devices. Mice were kept on regular 14 h/10 h light/dark cycle. Mice were weighed prior to and following placement of telemetry devices and on treatment days. Treatment began when mice were 8 weeks of age, with mice receiving treatments every other day for 2 weeks using dosing regimens described below and in Table 1. A minimum of 3 mice were included in each group with up to an additional 3 mice receiving telemetry devices. A full experimental design is shown in Fig. 1.
Schematic of experimental design. Following placement of telemetry devices in a subset of animals, mice were dosed every other day during the treatment phase (2 weeks). Dosing was conducted via gavage of a crude Cannabis extract, free feeding of whole Cannabis, or intraperitoneal injection (i.p.) of purified CBD/THC. Plasma and tissue were collected at study end
Selection of dosages
This pilot study was designed to determine if changes in neurochemicals, eCB, or physiology could be seen following repeated, oral dosing of high CBD/low THC in a whole Cannabis matrix in mice. Doses were based on the limited published reports that were available, to our knowledge, at study initiation, that utilized extracted Cannabis (Renard et al. 2017; Ressler and Nemeroff 2001; Richard et al. 2009) or purified THC/CBD (Roehler et al. 2022) specifically in mice. For example, Okon, et. al., administered 10 mg/kg-20 mg/kg per day of an organic extract of whole Cannabis buds for 28 days using oral gavage (Richard et al. 2009; Ruddick et al. 2006). Dosing in the study by Okon, et. al., was based on dry weight of Cannabis and phytocannabinoids concentrations were not measured. Dosing in a ratio of 10:1 CBD/THC was chosen based on the reported values of the NIDA whole Cannabis product. To our knowledge, at the time of study initiation, there had been no study that utilized whole, unextracted Cannabis for any type of intervention in mice; therefore, doses for whole Cannabis free feeding mimicked those used for gavage. Final concentrations of CBD/THC in the gavage and free fed mice were also limited by the volume or weight, respectively, of the final preparations that could be administered or consumed. Conversely, higher doses of THC and CBD administered in a single dose via i.p. had been shown to be safe in mice and hence higher doses were used for i.p. experiments (Roehler et al. 2022). The maximum dose of CBD was based on Deiana, et. al., who dosed animals with 120 mg/ml of purified CBD (Scherma et al. 2019).
Mouse telemetry
Telemetry devices (eMitters, Starr Life Sciences) were surgically implanted in mice at age 6 weeks and mice were given 7–10 days to recover from surgery. Mice were anesthetized using inhaled continuous flow isofluorane (1–3% isoflurane) for the duration of the surgery (approximately 10 min). A small, 1 cm incision was made in the abdomen at the ventral midline and the transmitter placed under sterile conditions into the abdominal cavity of the mouse. The abdominal wall fascia was closed using simple interrupted 4–0 sterile absorbable suture. 100 µL of 0.025% bupivacaine was placed topically on the incision site and the skin layer was closed with sterile wound clips. Mice were removed from anesthesia and monitored for recovery for at least one hour. Post-operative pain control was provided SQ using Buprenorphine at a dose of 1 mg/kg one time. Mice were monitored twice daily over the following 72 h for mobility, wound closure, and general appearance. Staples/sutures were removed 7 days post-operatively. Transmitters remained in the mouse for the duration of the study. Following the 7 days recovery period, temperature and heart rate readings were recorded every minute.
Preparation of C. sativa, C. sativa extracts, and CBD/THC
Approximately 250 mg of Cannabis was weighed into 16 × 125 mm borosilicate glass culture tubes (Fisher Scientific) fitted with PTFE/silicone-lined caps (VWR, Radnor, PA) and heat activated for 4 h at 120 °C on MULTIVAP Nitrogen Evaporator fitted with a heat block and culture tube adapters (Organomation, Berlin, MA). Aluminum foil was fitted over the heat block and tubes to retain heat. Activated Cannabis (50–150 mg) was weighed into TissueLyser tubes with a steel ball (Qiagen, Hilden, Germany). Tubes were centrifuged at room temperature and 18,000 × g for 2 min to prevent static movement of material. 200 proof ethanol was added to tubes at a ratio of 100 µL to 10 mg cannabis plant material. Cannabis was homogenized with a Qiagen TissueLyser LT bead mill for 5 min at 50 Hz and tubes were centrifuged at 0° C and 18,000 × g for 15 min. 20 µL of extract was reserved for targeted cannabinoid analysis in a 1.5 mL microcentrifuge tube at -20 °C. Remaining extract was used for mouse gavage.
Mouse gavage
Gavage solutions were prepared under sterile conditions in a Nuair Biological Safety Cabinet (Plymouth, MN). Ethanol extracted Cannabis was sterile filtered in Pierce spin cups (Thermo Scientific, Waltham, MA) and centrifuged for 15 min at 4 °C and 18,000 × g. Flow through was pooled in a sterile 50 mL Falcon tube (Fisher Scientific), capped and vortexed well to mix. Twenty microliters of extract was reserved for targeted cannabinoid analysis in a 1.5 mL microcentrifuge tube and stored at -80° C until analysis.
Low, Medium and High doses (2.5, 5 and 12.5 mL, respectively) of pooled sterile-filtered extract were dried in 2 mL microcentrifuge tubes (Fisher Scientific) under nitrogen at 55 °C in 1–2 mL increments. The stock pooled extract was vortexed for 5 s before each addition to dose tubes. Dried extracts were reconstituted in 239.6 µL of ethanol and vortexed twice for 15 s to mix. A control dose was made from ethanol without Cannabis extract. Doses were sterile filtered in Pierce spin cups as above and stored at -80 °C. Dose solutions were made daily by combining the ethanol extracts with Tween80:3% saline solution (1:1:18 v/v/v) in 1.5 mL microcentrifuge tubes, stored at 4 °C and then transported on wet ice to the vivarium. Mice were gavaged at rates of 0, 10, 20 and 50 mg CBD/kg every other day for 2 weeks. Mice were weighed each morning prior to dosing. Dose solutions were vortexed well and approximately 100 µL was drawn up into 1 mL plastic syringe (Fisher Scientific) fitted with a 20 G × 38 mm plastic feeding tube (Instech Laboratories, Plymouth Meeting, PA). Feeding tubes were inserted through the mouth into the stomach of each mouse and the solution was administered.
Mouse intraperitoneal injection
Intraperitoneal injection (i.p.) solutions were made under sterile conditions as above. A 200 mg powdered CBD standard was dissolved in 450 µL ethanol. Three 1,125 µL aliquots of the THC standard solution were combined with either 23.5, 117.2 or 234.4 µL of the CBD solution (Low, Medium and High dose, respectively) and evaporated to dryness in a Labconco CentriVap Concentrator (Kansas City, MO) at 45 °C. Dried residues were reconstituted in 225 µL ethanol and sterile filtered in Pierce spin cups (Thermo Scientific) as above. Mice were dosed at rates of 0, 10, 50 and 100 mg CBD/kg. THC concentrations were kept at 10 mg THC/kg, except in control group which was 0 mg/kg. Dose solutions were made fresh under sterile conditions by combining the ethanol dose solutions with the Tween80:3% saline mixture (1:1:18 v/v/v). Solutions were aliquoted daily, stored at 4 °C and then transported on wet ice to the vivarium. Dose solutions were vortexed well and approximately 100 µL was drawn up into 1 mL plastic syringe (Fisher Scientific) fitted with a 25 G needle and injected intraperitoneally.
Free feeding
Cannabis plant material was heat activated and homogenized as described above, except without the addition of ethanol. One cube of bacon flavored Nutra-Gel (Bio-Serv, Flemington, NJ) was chilled at -20 °C for 15 min. Free feeding doses were prepared at 7.5, 10 and 30 mg/kg/day by combining appropriate amounts of cannabis (8, 10, 45 mg) and Nutra-gel (4.3, 4.3, 5 g, respectively) in a large weigh boat placed on ice. Doses were homogenized thoroughly by hand with two disposable 25 mm plastic cell scrapers (Fisher Scientific) and re-chilled at -20 °C for 15 min. Aliquots of 100 mg were weighed into 1.5 mL microcentrifuge tubes and stored frozen at -80 °C until day of dosing. Control doses were prepared in a similar manner, without the addition of cannabis. Daily dose aliquots were removed the morning of dosing and transported to the vivarium on wet ice. A 100 mm petri dish lid was placed into each mouse cage at the beginning of the study to administer the dose on. Mice were dosed every other day for two weeks. Mouse chow was removed from cages 2 h prior to free-feeding on dose days. Each dose aliquot was placed in the petri dish for easy access to the mouse. Mice were visually monitored to ensure complete ingestion and doses were typically consumed within 2 min.
Dosing regimen
Two experiments were conducted to maximize the use of a limited number of telemetry devices (Table 1). Experiment 1 included a set of mice receiving crude Cannabis extract by gavage and a set of mice receiving purified CBD/THC by i.p. Experiment 2 included mice receiving whole Cannabis by free feeding and a second set of mice receiving purified CBD/THC by i.p. Dosing amounts of CBD and THC were based on published studies with the goal of administering non-toxic doses in the range of previously reported sub-therapeutic to therapeutic amounts of CBD (Ruddick et al. 2006; Schlienz et al. 2020). CBD and THC were measured in plant material and extracts using LC/MS/MS to confirm concentrations.
Plasma and tissue collection
Mice were euthanized two days after the final treatment. Blood was collected in 1.3 mL K3EDTA micro sample tubes (Sarstedt Inc., Nuembrecht, Germany) via cheek bleeding, inverted 5 × to mix, and immediately placed on ice. Blood was centrifuged at 3,000 × g for 30 min at 4 °C, within 30 min of collection. Plasma was aliquoted into 1.5 mL microcentrifuge tubes and stored at -80 °C until analysis. Whole brains were collected in 15 mL Falcon tubes (Fisher Scientific), flash frozen in liquid nitrogen and stored at -80 °C until analysis.
Phytocannabinoid, neurochemical and endocannabinoid analysis by liquid chromatography mass spectrometry
All standards and internal standards used for LC/MS/MS analysis of phytocannabinoids were purchased from Cerilliant (Round Rock, Texas, USA). Neurochemical standards and internal standards were acquired from various sources, including Cerilliant, Sigma-Aldrich (St. Louis, MO), Cayman Chemical (Ann Arbor, MI), and Cambridge Isotope Labs (Tewksbury, MA). All endocannabinoid standards were purchased from Cayman Chemical. All HPLC solvents and extraction solvents were HPLC grade or better. A list of all compounds included in the phytocannabinoid, neurochemical, and endocannabinoid assays is included in the Supplemental File 1. Only compounds found to be significant are included in the Results section; however, full results are available in the Supplemental Files 2–4.
Quantitation of cannabinoids in plant material and plant extracts
To assess extraction and filtration methods, a portion of the ethanol extract was analyzed prior to dosing mice. Portions of plant extractions were diluted by a factor of 10,000 in ethanol, transferred to a reduced surface activity/maximum recovery glass autosampler vial (Cornerstone Scientific, Wilmington, NC), and analyzed by LC/MS as described below.
Preparation of brain tissue homogenate
Brain samples were homogenized using a Qiagen TissueLyser LT (Qiagen, Hilden, Germany). Briefly, samples were placed into pre-chilled TissueLyser tubes containing a stainless-steel bead and a microliter volume of 1 × PBS solution equal to twice the tissue mass in milligrams was added to each sample. Samples were then homogenized at 50 Hz for 5 min followed by centrifugation for 10 min at 14,000 rpm and 4 °C. The supernatant was then collected and reserved for phytocannabinoid, neurochemical and endocannabinoid analysis.
Preparation of brain tissue and plasma for phytocannabinoid analysis
Proteins were precipitated from 25 µL of tissue supernatant or plasma by adding 25 µL of ice-cold methanol and 50 µL of the ice-cold internal standard solution (50 pg/µL each of Cannabidiol-D3, (-)-Δ9-THC-D3, ( ±)-11-Hydroxy-Δ9-THC-D3 in methanol) in a 1.5 ml microfuge tube, followed by vortexing and then incubating on ice for 15 min. The samples were then centrifuged for 10 min at 4 °C at 14,000 RPM and the supernatant was transferred to a reduced surface activity/maximum recovery glass autosampler vial for analysis.
Liquid chromatography mass spectrometry for phytocannabinoid quantitation
Quantitation of cannabinoids and neurochemicals was performed using reverse phase HPLC tandem mass spectrometry (LC/MS/MS). The HPLC system consisted of an Agilent 1290 autosampler (Agilent Technologies, Santa Clara, CA), an Agilent 1290 binary pump, and a 1200 series column compartment. Analysis buffers consisted of 0.1% formic acid in water (solvent A) and 1:1 acetonitrile: methanol (v:v, solvent B).
Two microliters of extracted plasma or 5 µL extracted brain was injected onto an Agilent Eclipse Plus C-18 2.1 × 50 mm 1.8 um analytical column with an Agilent SB C-18 2.1 × 5 mm, 1.8 um guard column using the following gradient at a flow rate of 0.4 mL/min: hold at 30% solvent A:70% solvent B from 0–4 min, then a linear gradient from 70–82% B over the next 3.5 min followed by an increase from 82–90% B from 7.5–8 min, then holding at 90% B for an additional 4 min. The analytical column was re-equilibrated at starting conditions for 3 min before the next injection.
Mass spectrometry analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer in positive ionization mode. The drying gas was 120 °C at a flow rate of 12 mL/min. The sheath gas was 325 °C at 12 mL/min. The nebulizer pressure was 50 psi. The capillary voltage was 3500 V. Data for exogenous cannabinoids were acquired in dynamic MRM mode using experimentally optimized collision energies obtained by flow injection analysis of authentic standards (Supplemental File 1: Table 1). Calibration standards for each cannabinoid were analyzed over a range of concentrations from 0.5 – 10,000 pg on column. Calibration curves for each lipid mediator were constructed using Agilent MassHunter Quantitative Analysis software. Samples were quantitated using the calibration curves to obtain the on-column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/ml. Levels of phytocannabinoids for several mice were below the method limit of quantitation (LOQ) and, therefore, results are expressed in peak areas.
Preparation of brain tissue and plasma for neurochemical analysis
Proteins were precipitated from 20 µL of brain tissue supernatant or plasma by adding 110 µL methanol and 10 µL of internal standard solution (Cambridge Isotope Laboratories, U13C metabolite yeast extract reconstituted in 3:1 ethanol:1 mM HEPEs pH 7.1 with adenosine-ribose-13C5 and creatinine-d3 added) in a 1.5 mL microfuge tube, followed by vortexing and then incubating at -20 °C for 10 min. The samples were then centrifuged for 10 min at 4 °C at 14,000 RPM and the supernatant was transferred to a reduced surface activity/maximum recovery glass autosampler vial for analysis.
Liquid chromatography mass spectrometry for neurochemical quantitation
Quantitation of neurochemicals was performed using Hydrophilic Interaction Liquid Chromatography (HILIC) HPLC tandem mass spectrometry (LC/MS/MS). The HPLC system consisted of an Agilent 1260 autosampler (Agilent Technologies, Santa Clara, CA), an Agilent 1260 binary pump, and a 1260 series column compartment. Buffer A consisted of 10 mM ammonium acetate adjusted to pH 9.0 with ammonium hydroxide, and buffer B consisted of 90:10 acetonitrile:water with 10 mM ammonium acetate adjusted to pH 9.0 with ammonium hydroxide. 5 µM of methylenediphosphonic acid was added to both buffers.
A total of 0.5 µL of extracted brain or plasma was injected onto an Agilent Poroshell 120 HILIC-Z 2.1 × 100 mm 2.7 um analytical column with the following gradient at a flow rate of 0.5 mL/min: hold at 100% solvent B for 2 min, 100%-80% solvent B from 2–12 min, 80%-60% solvent B from 12–13 min, hold at 60% B from 13–15 min, 60%-100%B from 15–16 min. The column was then re-equilibrated at 100%B for 4 min before the next injection.
Mass spectrometry analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer in positive ionization mode. The drying gas was 130 °C at a flow rate of 15 mL/min. The sheath gas was 350 °C at 12 mL/min. The nebulizer pressure was 35 psi. The capillary voltage was 3000 V. Data for neurochemicals were acquired in dynamic MRM mode using experimentally optimized collision energies obtained by flow injection analysis of authentic standards (Supplemental File 1: Table 2). Calibration standards for each neurochemical was analyzed over a range of concentrations from 0.45– 235 pg on column for most of the target analytes, with epinephrine, metanephrine, normetanephrine, norepinephrine and 5-hydroxyindole-3-acetic acid from 4.5–2350 pg on column. Calibration curves for each neurochemical was constructed using Agilent MassHunter Quantitative Analysis software. Samples were quantitated using the calibration curves to obtain the on-column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/mL or pg/mg.
Preparation of brain tissue and plasma for endocannabinoid analysis
Proteins were precipitated from 50 µL of brain tissue supernatant or plasma by adding 220 µL methanol and 30 µL of internal standard solution (Arachidonoyl ethanolamide-d4 and oleoyl ethanolamide at 200 pg/ µL, 2-arachidonoyl glycerol-d5 at 2000 pg/µL in methanol) in a 1.5 mL microfuge tube, followed by vortexing for ~ 10 s. The samples were then centrifuged for 10 min at 4 °C at 14,000 RPM and the supernatant was transferred to a reduced surface activity/maximum recovery glass autosampler vial for analysis.
Liquid chromatography mass spectrometry for endocannabinoid quantitation
Quantitation of endocannabinoids was performed using reverse phase HPLC tandem mass spectrometry (LC/MS/MS). The HPLC system consisted of an Agilent 1260 autosampler (Agilent Technologies, Santa Clara, CA), an Agilent 1260 binary pump, and a 1260 series column compartment. Buffer A consisted of 0.1% acetic acid in water and buffer B was 90:10 acetonitrile and isopropyl alcohol.
2 µL of extracted brain or plasma was injected onto an Agilent Eclipse Plus C18 2.1 × 150 mm 1.8um analytical column with the following gradient at a flow rate of 0.3 mL/min: 30%-45% solvent B from 0–2 min, 45%-79% solvent B from 2–2.5 min, hold at 79% solvent B from 2.5–11.5 min, 79% B-100% B from 11.5–12 min, hold at 100% B from 12–16 min, 100% B-30% B from 16–20 min. The column was then re-equilibrated at 30% B for 2 min before the next injection.
Mass spectrometry analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer in positive ionization mode. The drying gas was 230 °C at a flow rate of 15 mL/min. The sheath gas was 400 °C at 11 mL/min. The nebulizer pressure was 35 psi. The capillary voltage was 4000 V. Data for endocannabinoids were acquired in dynamic MRM mode using experimentally optimized collision energies obtained by flow injection analysis of authentic standards (Supplemental File 1: Table 3). Calibration standards for each endocannabinoid were analyzed over a range of concentrations from 0.025– 50 pg on column for most compounds, with 2-linoleoyl glycerol at 0.25–500 pg on column and 2-arachidonoyl glycerol at 2.5–5000 pg on column. Calibration curves for each endocannabinoid were constructed using Agilent MassHunter Quantitative Analysis software. Samples were quantitated using the calibration curves to obtain the on-column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/mL or pg/mg.
Telemetry data processing
Processing of telemetry data was performed using Vital View software (Starr Life Sciences) according to the manufacturer’s suggestions. For temperature and heart rate data, missing values were first imputed by using the value just prior to the missing value. Following imputation, data were reduced by averaging values to obtain 6 data points per hour. Data were analyzed across time windows of 1 h increments, with windows designated as pre-dose, dose, post-dose 1, post-dose 2, post-dose 3, post-dose 4, post-dose 5, and post-dose 6. Therefore, a total of 8 h of data were considered.
Statistics
For body weight analysis, a 2-way ANOVA was conducted (Prism GraphPad) for individual treatment groups. For phytocannabinoid analysis, a 1-way ANOVA followed by Tukey’s post-hoc analysis was used for pairwise comparisons between treatment groups (Prism GraphPad). For each neurochemical or endocannabinoid analyte, a 1-way ANOVA followed by Fischer’s LSD post-hoc analysis was used for pairwise comparisons between treatment groups (Prism GraphPad). For several of the neurochemical or endocannabinoid analytes, the overall p-value from the 1-way ANOVA was significant, but after a Tukey correction, none of the post hoc comparisons reached statistical significance. Because this is a pilot study, we chose to use a Fischer’s LSD post-hoc analysis instead of Tukey for the neurochemical or endocannabinoid analyte to better facilitate the identification of potential trends within the data. For heart rate and body temperature analyses, 2-way ANOVAs were conducted. A separate analysis of heart rate and body temperature of mice during the predose window confirmed no differences between groups prior to dosing ( p = 0.106).